
Jose L. Toro, Ph.D., new Principal Consultant at Lachman Consultant Services, Inc.
We are pleased to announce that Jose L. Toro has accepted the position of Principal Consultant in the Compliance Practice at Lachman Consultants, effective November 13, 2017. Dr. Toro has extensive R&D Quality, and Quality Operations experience in the pharmaceutical industry. He specializes in the transformation of Quality and Technical Services organizations including Quality Systems, […]

Ricki Chase, M.S., Director, Lachman Consultants, to Present at American Pharma Outsourcing Summit
Ricki Chase, Director, Compliance Practice, will be presenting at the American Pharma Outsourcing Summit taking place September 27th and 28th, 2017 at the Doubletree by Hilton Boston North Shore. Ricki will be presenting on the Regulatory Hazards of Outsourcing, and will cover the following areas: Approving products on a batch-by-batch basis Establishing laboratory controls Training […]

UGA’s Wided Najahi-Missaoui Granted First Women in Pharma Scholarship (iSpeak blog, ISPE, September 8, 2017)
Wided Najahi-Missaoui, MS, PharmD, doesn’t know the meaning of the word “can’t.” Since arriving in the United States from Tunisia in 2000, she has earned two graduate degrees (a close friend says she collects degrees like others collect stamps), and accolades. Her mentor, Dr. Michael Griffith Bartlett, who is Professor and Director–BS Program at the […]

David G. Lonza, B.S. Engineering, new Head of EU at Lachman Consultant Services, Inc.
We are pleased to announce that David Lonza has accepted the position of Head of EU at Lachman Consultants, effective September 1, 2017. David Lonza joined Lachman Consultants in August 2016, in the role of Deputy Advisor to the President / CEO – Strategic Initiatives. David’s expertise includes servicing pharmaceutical / biopharmaceutical / biotech clients […]

Article published in Contract Pharma by James Davidson, PhD
James Davidson, PhD, Vice President, Science and Technology practice, Lachman Consultants recently authored an article in Contract Pharma. The article, “Data Integrity Guidance Around the World”, reviews guidance issued by worldwide authorities (FDA, MHRA, PIC/S, and TGA) with regards to data integrity controls and the overall framework for a data governance system. The article can […]

Lachman Consultants Participates in the ISPE/FDA/PQRI Conference
A few weeks ago, Lachman Consultants participated in the ISPE/FDA/PQRI Quality Manufacturing Conference (“Conference”) in Alexandria, VA. For those that have never attended, this is a particularly dynamic meeting for those that are involved in manufacturing in the regulated industry. There were a myriad of presentations and workshops with members of FDA and industry professionals, […]
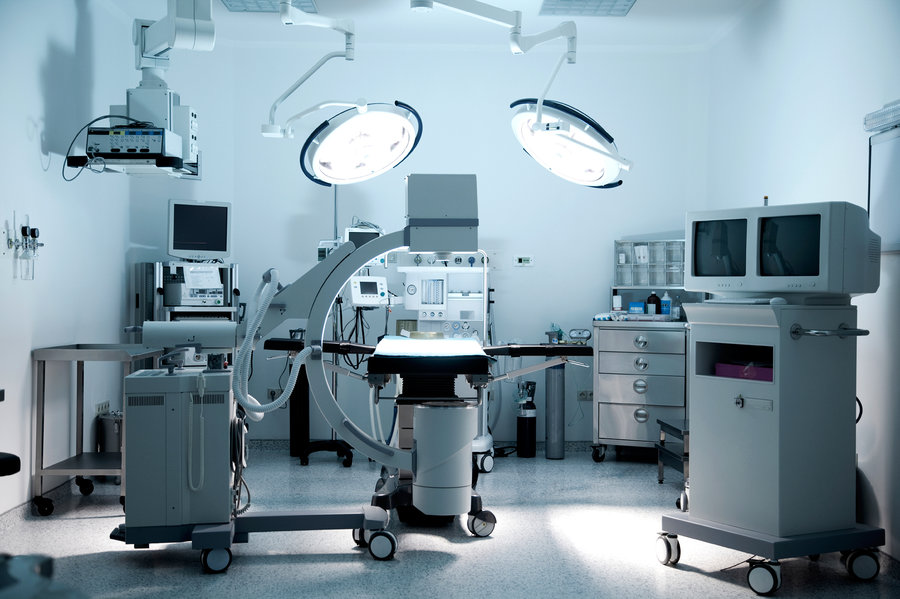
White Paper: Don’t Be “Device-ive”; Keep Up or Be Left Behind
On May 8, 2017, the US FDA’s Center for Radiological Health announced that it will be establishing a new digital health unit under the Office of the Center Director. This is just the latest step the Agency is taking to try to keep pace with the quickly evolving and rapidly growing digital medical device industry. […]

Linda Evans O’Connor Presents in Singapore
On April 27, 2017, Linda Evans O’Connor, Head of Business Processes and Regulatory, presented at the 2nd Healthcare Asia Pacific Summit 2017 in Singapore. She presented on international regulatory trends, FDA enforcement actions for foreign and domestic firms, the new US Administration and effect on expected guidances, and quality culture.

James Davidson, Ph.D. to Present at the Food and Drug Law Institute Annual Meeting
James Davidson, Ph.D., Vice President, Science and Technology, will be moderating a session at the Food and Drug Law Institute Annual Meeting in Washington, D.C. The session, “Responding to and Remediating Data Integrity Issues in a 483 or Warning Letter’, will take place on Friday, May 5 at 12:30 PM in the Ronald Reagan Building […]

Jean Poulos, M.S, M.B.A., new Principal Consultant at Lachman Consultant Services, Inc.
We are pleased to announce that Jean Poulos has accepted the position of Principal Consultant in the Regulatory Practice at Lachman Consultants, effective April 10, 2017. Ms. Poulos is a seasoned Regulatory professional with more than 25 years of combined quality assurance, quality control, regulatory affairs and compliance enforcement experience in the pharmaceutical / biotechnology […]



