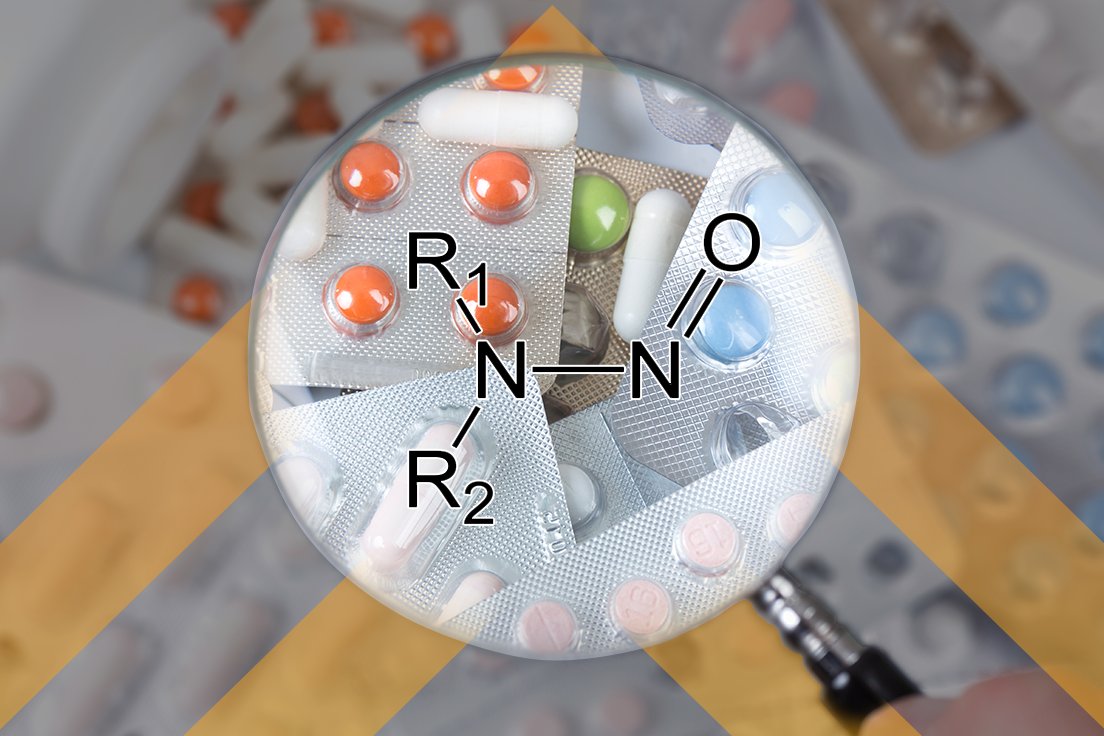
FDLI Session Addresses the Nitrosamine Issue
Hundreds of FDA staff, industry professionals, lawyers, and others gathered in Washington, D.C. on May 17th and 18th to participate in the 2023 Food and Drug Law Institute (FDLI) Annual Conference. The agenda covered a broad range of current regulatory and legal issues facing the food, drug, medical device, biologics, and tobacco industries. Lachman Consultant Services […]






