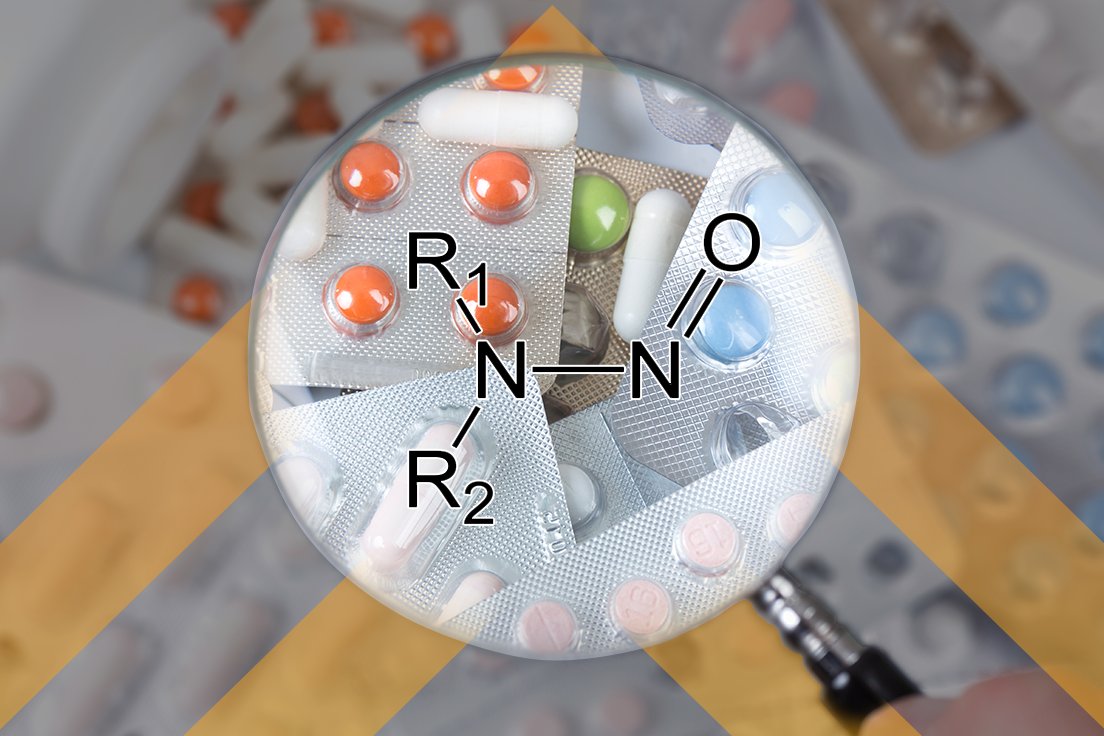
The FDA’s Spotlight on CDER Science – Nitrosamines and the Acceptable Intake Approach
In the latest CDER Science Spotlights, available (here), the FDA provides some insight into the development of the Carcinogenic Potency Categorization Approach (CPCA) to determine recommended acceptable intake limits of N-nitrosamine impurities in drug products. The latest revision of the Nitrosamine Impurity guidance updated in September 2024 (here) provided better integration of the overall assessment […]