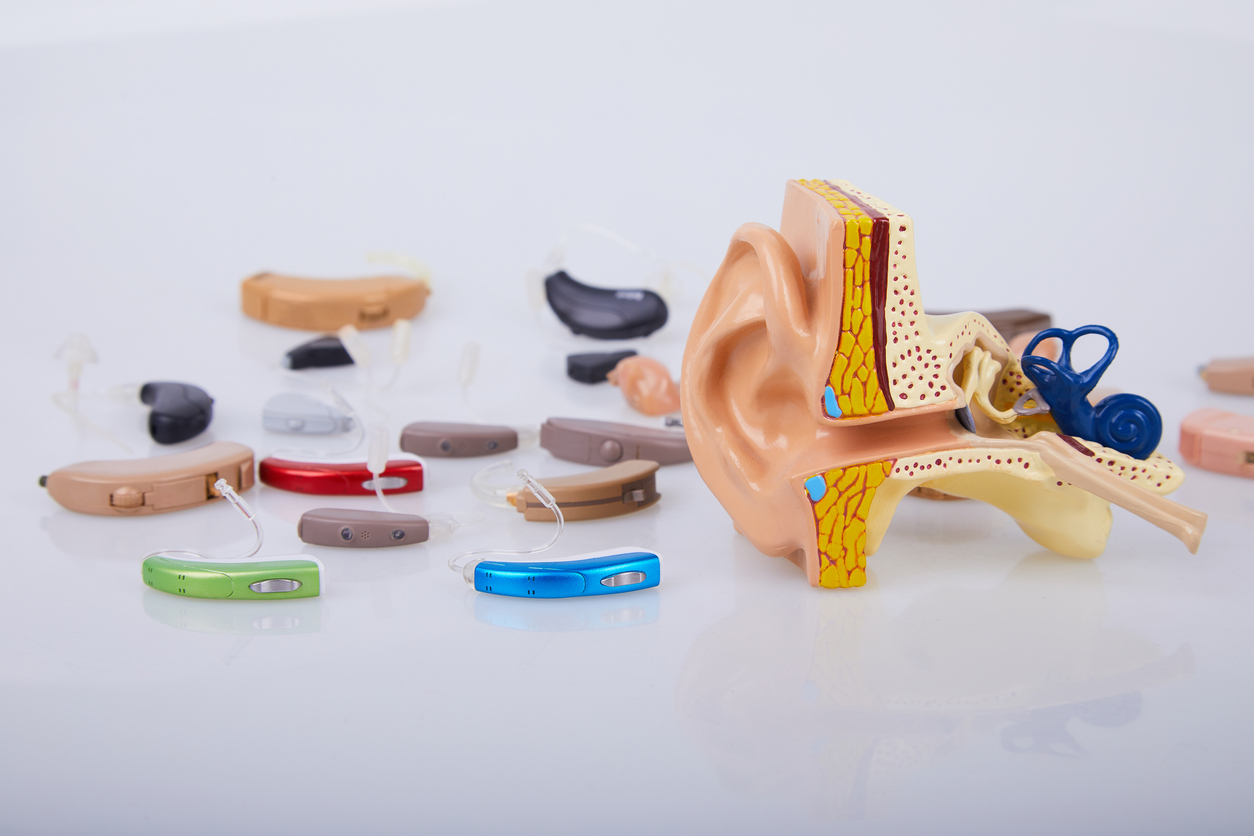
I Hear You – But Do I Understand You? Hearing Aids to Go OTC!
Tomorrow, FDA will issue a revised draft guidance on Hearing Aids and Personal Sound Amplification Products (PSAP) (here), and is also publishing a proposed rule for the over the counter (OTC) classification of hearing aids (HA) and PSAP (here) entitled “Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids”. These are the prepublication […]