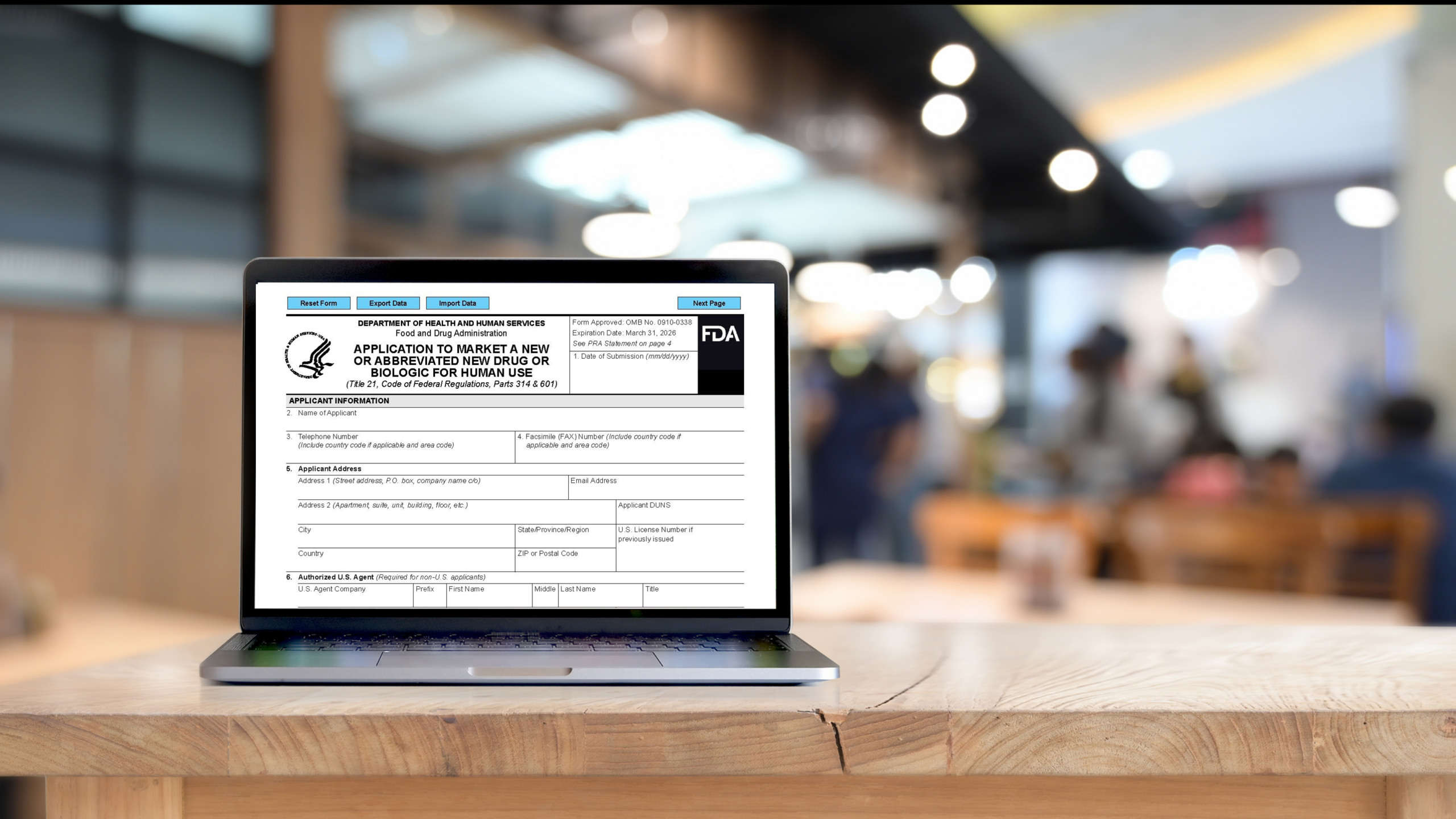
Are You Using the New FDA Form 356h?
The new Form FDA-356h was released in July 2023. The FDA’s expectation is that the newly revised form (which has an expiration date of March 31, 2026) should be utilized when submitting new drug applications, abbreviated new drug applications, and biologics license applications for human use. The new form can be downloaded from a link that […]












