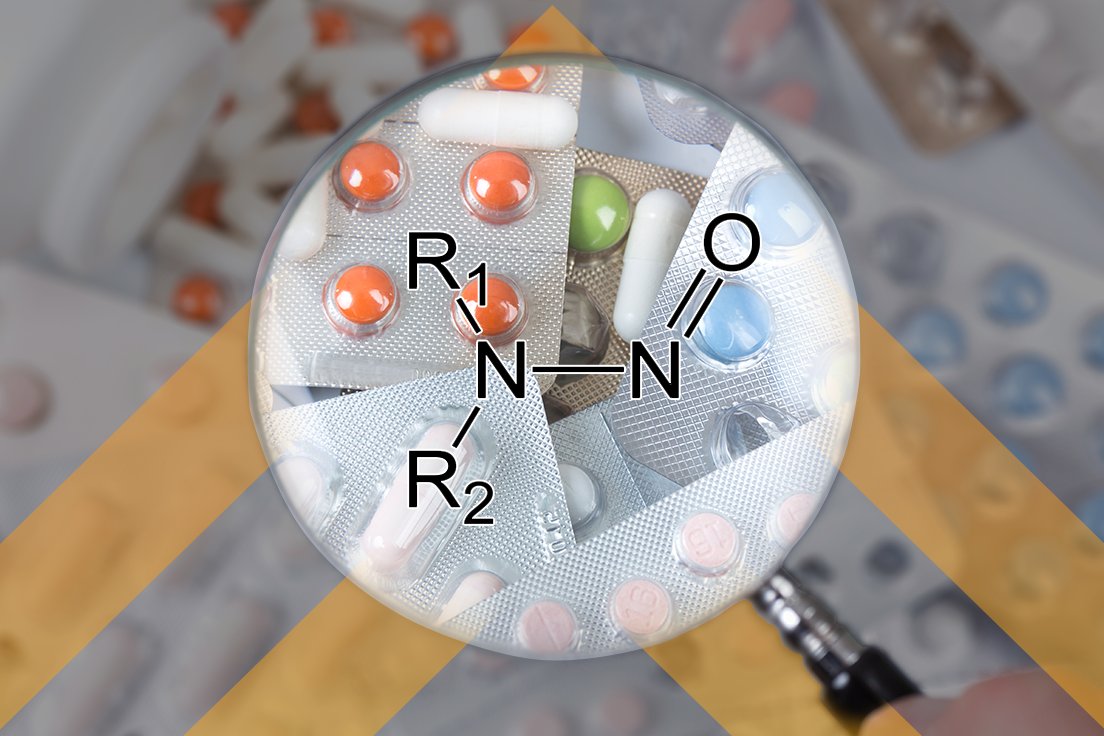
Establishing a CCS Foundation for Cleaning Validation
Bring up the recently updated and finalized EU Annex 1 – Manufacture of Sterile Medicinal Drug Products (here) in polite pharmaceutical water cooler conversation and a frequent topic that comes up is the Contamination Control Strategy (CCS) language. Buzzwords abound for a “holistic”, “global”, or “wholesale” CCS to be incorporated into all elements of aseptic […]