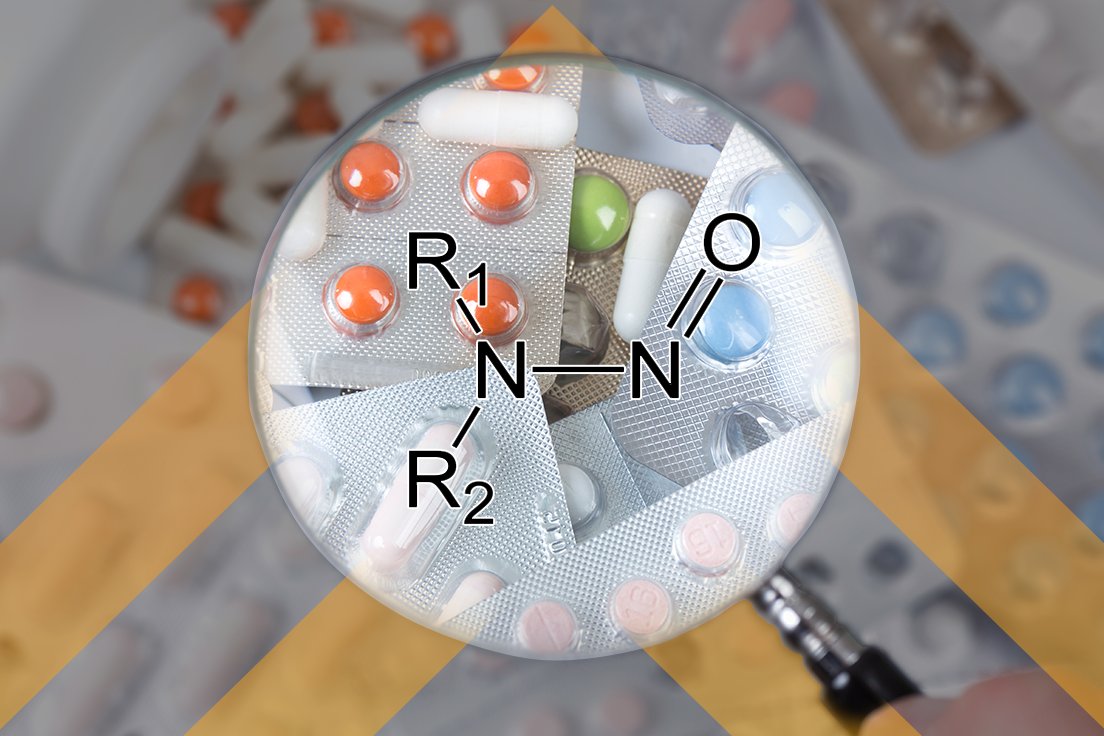
Nitrosamines Harmonization: The Conversation Continues…
As Bob has previously blogged on the highlights from AAM day by day, let me take a moment to dive a bit deeper into one of the sessions. As an attendee of this meeting for several years, one of the best things about AAM’s GRx+Biosims is that sessions at this meeting often offer a mix […]