With the development of new technologies and drugs, the need for certain products to be presented as combination products for administration is becoming more and more commonplace. According to the FDA FAQs page on Combination Products (here), “Combination products are typically marketed under a marketing authorization type associated with the constituent part that provides the primary mode of action (PMOA) for the combination product (i.e., a new drug application (NDA) or abbreviated new drug application (ANDA) if it has a drug PMOA, a biologic license application (BLA) if it has a biologic PMOA, or a premarket approval application (PMA) de novo certification, or premarket notification (‘510(k)’) if it has a device PMOA). A single marketing application is generally sufficient for a combination product. In some cases, however, a sponsor may wish to submit separate marketing applications for different constituent parts of a combination product, and FDA may consider this permissible.” Combination products can be pursued as physical combinations, products packaged together, or products separately packaged but intended to be used together.
There are several issues that may arise when dealing with combination products, but this blog is going to address one specific issue, the challenge of coordinating the approval of your drug and device when separate marketing applications are submitted for the different constituent parts and the products are not a physical combination (pre-filled, for example).
For sponsors that have sought approval of a combination product, you are likely aware that it can be very difficult to coordinate approval of the drug product application and the device clearance. Not only very difficult, but likely a true stumbling block that may cost your company additional resources, both in time and cost. In fact, this blogger would suggest that it is common knowledge that there is a disconnect at the FDA that will likely require “resubmission” of the device for clearance, even multiple times. Not only does resubmission of the device component require a significant amount of resources at a company and at the FDA, but it also requires a MDUFA fee for each resubmission. At the current rate for a 510(k) submission, this is $21,760 for each resubmission, and oftentimes the only reason for resubmission is the lack of an approved drug product for reference (in other words, the device is otherwise ready for clearance, but it cannot be cleared because the associated drug product is not ready for approval).
Further complicating the situation, in May 2024, the FDA issued an updated alert regarding cross-compatibility issues with autoinjector devices for a specific product, glatiramer acetate injection (here and, as previously reported in Lachman Consultants’ blog, here). This newly issued alert now requires updated labeling to include a new warning that using an autoinjector that is not (or, presumably, has not been demonstrated to be) compatible with a specific glatiramer injection product should not be used as it could lead to medication errors. In the case referenced above, if the autoinjector seeking clearance is not used in any other approved drug product, then it cannot be cleared as clearance requires that there be an approved drug product with which it will be used. However, as an autoinjector is to be identified in the labeling of the glatiramer injection drug product’s Prescribing Information (PI), this requires that the autoinjector device be cleared prior to the drug product’s approval.
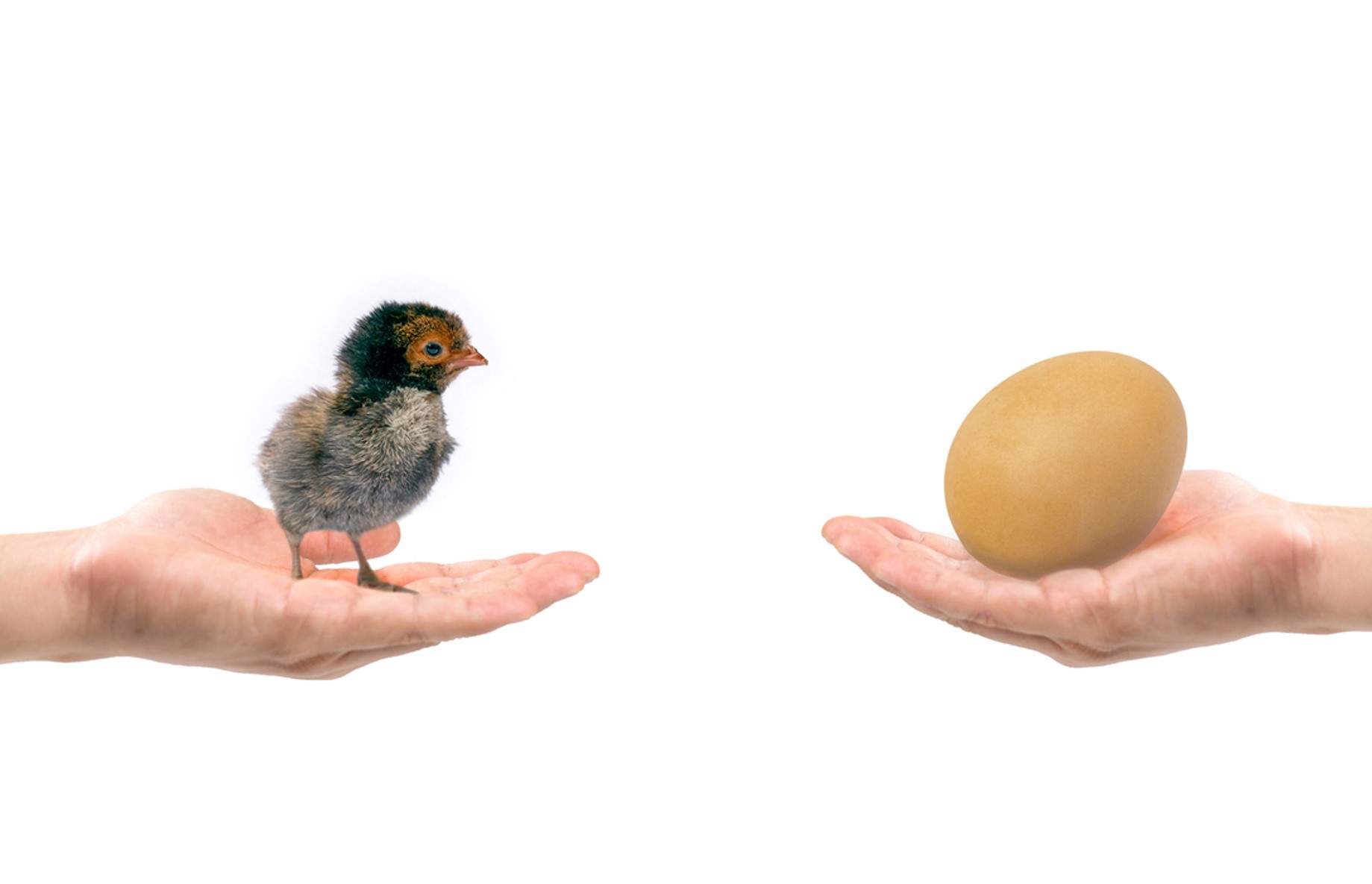
This is a case of the chicken and the egg, which makes industry ask, “How can I get these cleared and approved at exactly the same time?” This coordination of two applications to separate Centers by a single applicant is increasingly difficult, particularly since the advent of late-cycle amendments (LCAs), which are occurring under GDUFA III (see Lachman blog here) and have made drug product approvals a moving target.
This situation needs to be addressed by the FDA. CDER, CBER, and CDRH should create a mechanism to coordinate reviews and/or approvals of applications cross-referenced for use together. This blogger recommends that this topic be brought to the table during the User Fee discussions for the next round of PDUFA, GDUFA, and MDUFA agreements. At minimum, in the future, industry and the FDA should be able to agree that submission of an additional MDUFA fee should not be required under these circumstances.