Some of the big-ticket items mentioned in the FY 2023 report are Office of Compliance (OC) actions taken regarding the “contamination from diethylene glycol and ethylene glycol (DEG/EG), potentially harmful eye products, and mitigating risks from drug shortages while ensuring the safety our drug supply.” These issues caused the OC to “pivot” from its business as usual quickly to protect the public.
The OC reported its usual activities by the numbers for different aspects of its work. For Policy and Procedure, the OC mentioned that it issued thirteen guidance documents, issued forty-four immediate notices regarding fraudulent healthcare products, gave 110 presentations to stakeholders, provided ten presentations on the FDA’s YouTube channel, and provided training sessions to 4,232 stakeholders on compounding.
Relative to compliance action in FY 2023, the OC issued 170 warning letters, monitored 264 drug recall events (totaling 1,178 recalled drugs), oversaw two consent decrees for quality violations, issued ninety-five import alerts to help prevent various foreign facilities from importing drugs into the U.S., and took action to deactivate 19,265 drug listings from the FDA’s Drug Registration and Listing System.
Compliance review activities included 300+ documents shared with foreign regulatory contacts, thirty-two pre-notice of noncompliance letters issued for ClinicalTrials.gov violations, 218 drug manufacturing inspection classification letters issued, 9,778 Electronic Certificates of Pharmaceutical Product issued to provide documentation of facilities’ compliance with FDA standards, and 100% of clinical inspection summaries issued by agreed-upon goal dates for the various user fee programs.
The report has various charts and discussion sections addressing its activities. One chart, reproduced below, indicates the types of issues that resulted in warning letters:
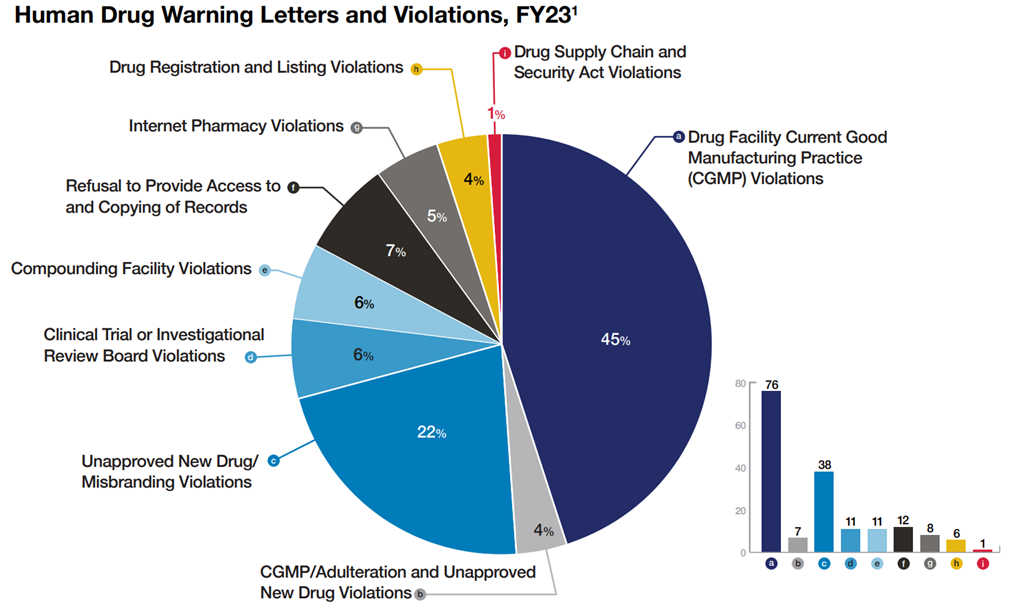
The full Compliance Report, chock full of other statistics and information, can be found here.




