On the Federal Register (FR) prepublication page today, the FDA outlined the new fee schedule for generic drugs under the GDUFA program for FY 2024. Make sure you have a bottle of Maalox nearby before you read the rest of this blog as the increases, especially for the program fees, might cause you some agita.
Generic Drug User Fees For 2024
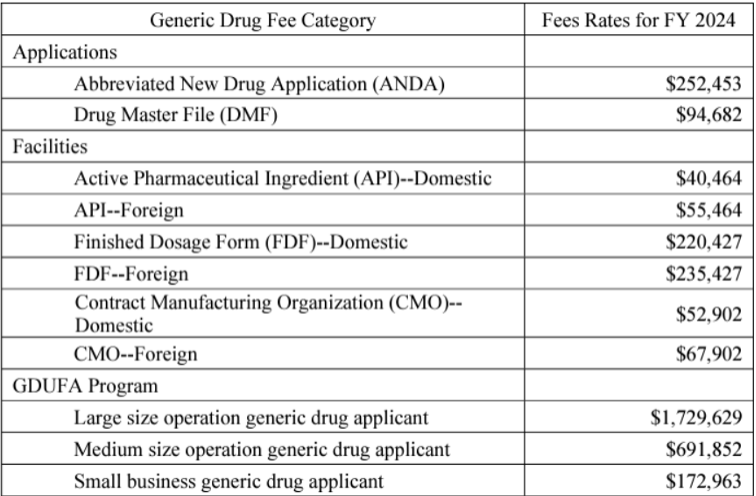
Fees for ANDA and DMF applications increased by $11,871 and $16,389, respectively, over last year’s fees, while the fees for API manufacturers went up by $2,920 for both domestic and foreign facilities.
FDF manufacturer fees increased by $7,293 for both foreign and domestic sites, while CMO fees increased by only $1,750 for both foreign and domestic facilites.
Program fees jumped by $109,073 for largesize applicants, followed by jumps of $43,630 for mediumsize and $10,907 for smallsize business applicants. The FR notice says, “[F]actoring in all the variables, we estimate there will be 205 applicants in the small business tier, 68 applicants in the medium size tier, and 80 applicants in the large size tier for FY 2024.”
The Federal Register prepublication notice can be found here; you can read about how the FDA set the 2024 fees, and its projection of inflation and workload that had it arrive at these figures. Of note, the government is projecting 801 ANDAs in FY 2024, which, when we look at the current number of projected ANDAs for FY 2023 with eight months of data for ANDA receipts, projects only 768 new ANDA submissions if the current pace continues for the next four months. FY 2022 saw the submisson of 857 ANDAs, while 2021 saw just 809 new ANDAs submitted. Now look at Congress trying to cut prescription drug costs while most years raising fees to firms that submit applications that the FDA receives for review. Who is going to absorb the increases? As generic margins continue to fall with price pressure from PBMs, wholesalers, and insurance companies, it will ultimately be the patients who will have to pay for these increases! We will have more news on 2024 fees for the other UFA programs shortly.




