In the prepublication of the Federal Register this morning, the FDA announced the Animal Drug User Fee Rates (here) and the Animal Generic Drug User Fee Rates (here). Some of the fees increased significantly while others decreased. Fee variations are based on the projected number of applications, establishments, and products, as well as inflationary calculations. A description of how each fee was calculated appears in the respective FR Notices.
The new rates are provided in the following tables.
Animal Drug User Fee Rates and Payment Procedures for Fiscal Year 2023
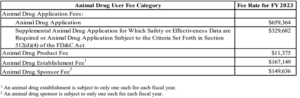
Some of the FY 2023 fees have risen considerably as the FY 2022 rates for NADAs were $580,569 and supplements with clinical data were $290,284, higher by $78,795 and $39,398, respectively. The FY 2022 animal product fee of $10,787, animal establishment fee of $155,220, and animal drug sponsor fee of $137,791 increased modestly by $588, $11,929, and $11,845, respectively.
Animal Generic Drug User Fee Rates and Payment Procedures for Fiscal Year 2023

FY 2022 fees for AANDAs were $548,628 and applications subject to 512(d)(4) were $274,314, higher last FY by $53,645 and $26,822), respectively. Product fees for FY 2022 were $17,513; the reported fee for 2023 is higher by a modest $1,368. And, finally, the 100%, 75%, and 50% sponsor fees for FY 2022 were $234,297, $175,723, and $117,149, lower last FY by $49,573, $37,180, and $24,786, respectively.
We will need to wait for the passage of the other UFA legislation before we see those user fee rates because the programs above have one year left on their authorizations. Negotiations for these programs will begin in FY 2023.




