The FDA announced today the addition of 25 new bioequivalence (BE) recommendations and has issued revisions of 24 previous recommendations. FDA continues its efforts in getting ahead of the curve trying to issue BE recommendations for “(1) new chemical entities and (2) approved on or after October 1, 2017, at least 2 years prior to the earliest lawful ANDA filing date” in an effort to aid in ANDA product development at the earliest possible date.
The list of new and updated recommendations can be found here. The actual recommendations can be found here. The following 2 tables are extracted from the FR notice cited above.

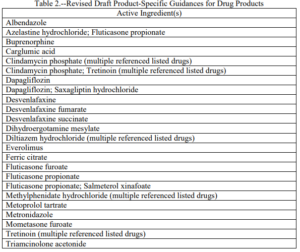
The problem associated with the revised recommendations is that there is no discussion of the changes that have been made from the previous versions making it difficult for applicants to readily identify changes that may impact soon to be submitted or pending ANDAs. We implore the Agency to rectify this important change.




