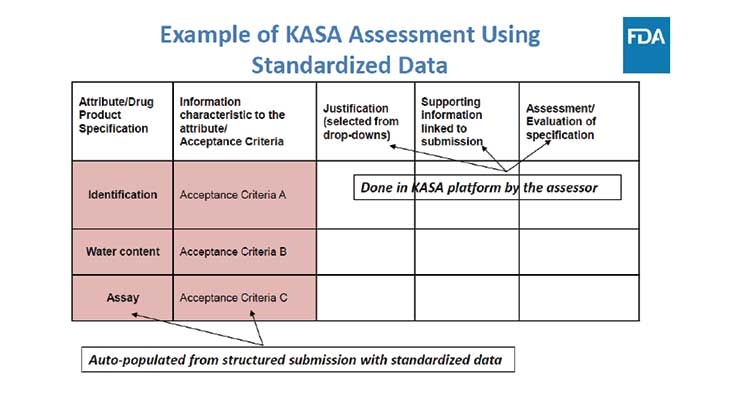
Contract Pharma published an article in April addressing the need for a modernized version of “our generic drug review process.” FDA anticipates a data-based assessment will “improve consistency, transparency, communication, and objectivity of regulatory actions as well as knowledge management within the Agency.”
Knowledge-aided Assessment & Structured Application (KASA) is in support of the Generic Drug Review and its first step is to transform the system from “a text-based to a data-based assessment.”
The Office of Pharmaceutical Quality (OPQ) drug review process is “a 20th Century approach” which is not suitable for this digital era. Therefore, a new system for drug quality assessments will be developed in alignment with the President’s Budget for pharmaceutical drugs.