In two separate Federal Register notices (here for medical devices and here for outsourcing facilities), the FDA announced the user fee amounts for FY 2020. The FDA notes that the fees are effective on October 1, 2019 (the beginning of the 2020 FY) through September 30, 2020.
The medical device fees are provided in the charts below, but in its discussions of the small business fees, the Agency notes that there is no lower small business fee for the establishment fee.
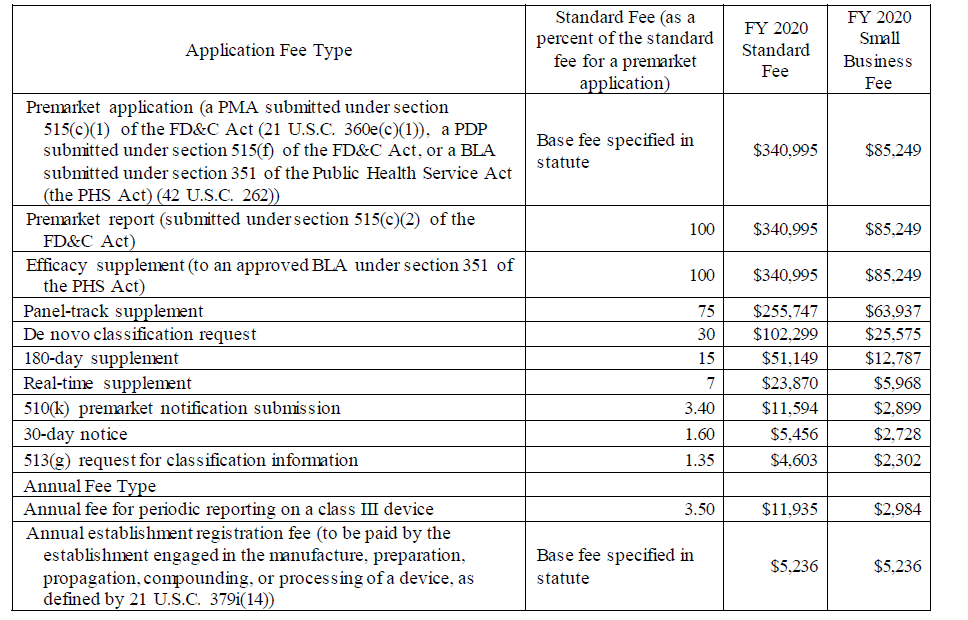 The notice describes how to apply for the small business MDUFA fees and provides information on how the fees for FY 2020 were calculated based on the expected number and types of applications, establishments, and inflation adjustments as defined by law.
The notice describes how to apply for the small business MDUFA fees and provides information on how the fees for FY 2020 were calculated based on the expected number and types of applications, establishments, and inflation adjustments as defined by law.
The establishment and reinspection fees related to entities that compound human drugs and elect to register as outsourcing facilities (OF) under the Federal Food, Drug, and Cosmetic Act (FD&C Act) are outlined in the second notice cited above. The fees apply to registered outsourcing compounding facilities. This notice, like the MDUFA notice, provides information on how the fees were calculated, how many OF the FDA expects, and how the inflation calculation is applied to each fee.
The fees are provided below:
Outsourcing Fees for FY 2020
 Please refer to the Federal Register notices for additional information.
Please refer to the Federal Register notices for additional information.




