Required by Section 914 of the Food and Drug Administration Amendments Act of 2007, the annual report of the impact of 505(q) petitions and petitions for stay of action issued this last Thursday. This report discussed the number of such petition and describes any delays in approval that such petitions may have caused. The report covers FY 2017.
The report is required to contain the following information:
- The number of abbreviated new drug applications (ANDAs), 505(b)(2) applications, and 351(k) applications approved during the reporting period;
- The number of such applications that were delayed by 505(q) petitions;
- The number of days by which the applications were delayed; and
- The number of 505(q) petitions that were submitted during the reporting period.
The Agency reports that 763 ANDAs, 57 505(b)(2) applications, and three 351(k) applications were approved during FY 2017. Interestingly, there were no delays in approval for ANDAs or 351(k) applications caused by such petitions and only one 505(b)(2) application was reported as being delayed. The Agency reported that it received 25 505(q) petitions during FY 2017.
While petitions did not seem to directly or significantly delay the approval of applications over the period of the 10 years of reporting, the FDA is still concerned about the drain on resources necessary to respond to such petition even though most “do not raise valid scientific issues” and are, according to the FDA report, submitted “primarily to delay approval of competing products.”
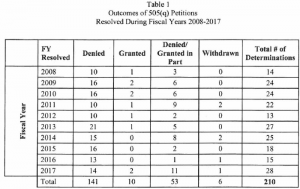
The Agency notes that “as of September 30, 2017, 141 of the petitions (approximately 67%) have been denied. Another 53 petitions (approximately 25 percent) have been denied in part and granted in part. Only 10 petitions (approximately 5 percent) have been granted. An additional 6 petitions (approximately 3 percent) were voluntarily withdrawn by the petitioner.”
The chart below provides addition statistics on the petitions submitted since the reporting requirement went into effect. Because the statute requires FDA to prioritize responses to their petitions above everything else, the resources devoted to responding to them have an adverse impact on other work the Agency could have addressed even through there has been limited impact on the applications that were subject to the petitions. A complete copy of the report can be found here.