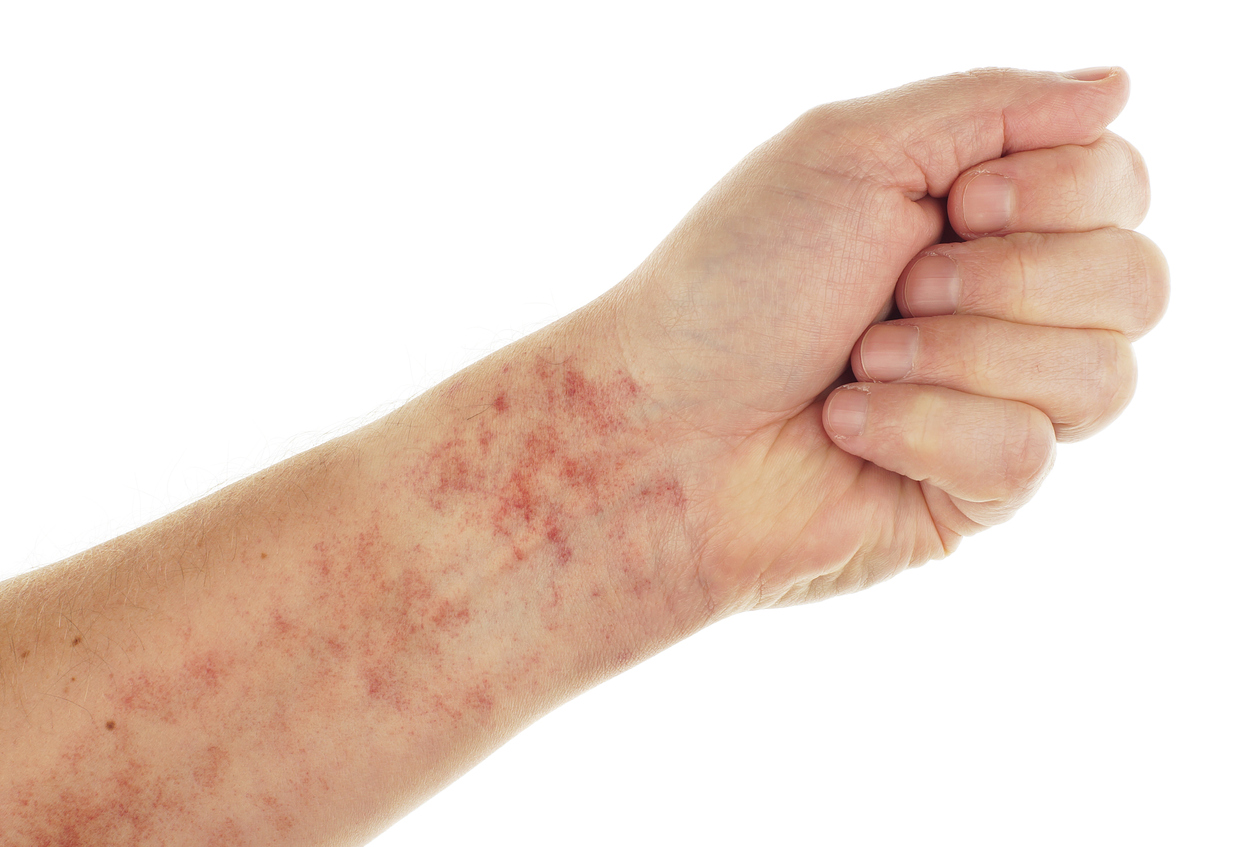
The FDA issued a safety warning on Thursday afternoon regarding the potential for rare but serious adverse reactions (including anaphylaxis, hives and severe rash) for a common skin sanitizing agent. The warning focuses on chlorhexidine gluconate which is available both as a prescription and over-the-counter product for use as a skin sanitizer. In addition, the product is also available in an oral for use in gum disease products but those products already have the warning.
The Agency identified 52 serious cases of anaphylaxis in a 46-year period between January 1969 and June 2015, but the alarm is sounding because more than half of the cases were reported after 2010.
Labeling for prescription and OTC products that do not already carry the warning will be updated shortly. The full FDA Safety Communication can be found here.